- Introduction: The Biogenic Amines Urine LC-MS/MS Kit is designed for the quantitative determination of biogenic amines in urine samples using liquid chromatography-tandem mass spectrometry (LC-MS/MS) technology. This protocol provides step-by-step instructions for the proper usage of the kit.
- Kit Components:
- Biogenic Amines Standard Mix
- Internal Standard Solution
- LC-MS/MS Grade Solvents
- Sample Preparation Tubes
- LC-MS/MS Vials and Caps
- User Manual
- Precautions:
- Read the user manual thoroughly before starting the assay.
- Handle all reagents with caution and follow safety guidelines.
- Use proper personal protective equipment, including lab coats, gloves, and safety goggles.
- Ensure proper disposal of all waste materials according to local regulations.
- Sample Preparation:
- Collect urine samples in clean, sterile containers.
- Label each sample with a unique identifier.
- Prior to analysis, centrifuge the urine samples at 3000 rpm for 10 minutes to remove any particulate matter.
- Transfer the supernatant to sample preparation tubes provided in the kit.
- Calibration:
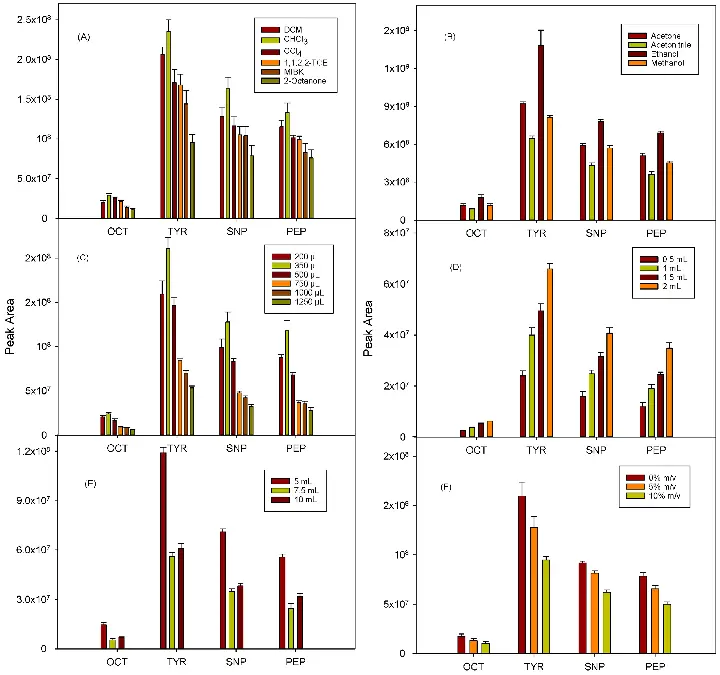
- Prepare calibration standards using the Biogenic Amines Standard Mix according to the concentrations specified in the user manual.
- Dilute the standards with LC-MS/MS grade solvents to obtain desired concentrations.
- Prepare a calibration curve by plotting the peak area ratio of analytes to internal standard against the concentration of standards.
- LC-MS/MS Analysis:
- Inject the prepared calibration standards and urine samples into the LC-MS/MS system according to the instrument's operating manual.
- Set up the LC-MS/MS system parameters as recommended in the user manual.
- Run the chromatographic separation using the provided LC-MS/MS grade solvents and column conditions.
- Data Analysis:
- Analyze the chromatographic data using the appropriate software provided by the LC-MS/MS system manufacturer.
- Quantify the concentrations of biogenic amines in urine samples by comparing peak areas to the calibration curve.
- Report the results in the units specified by the user manual.
- Quality Control:
- Perform quality control checks using provided standards and controls to ensure the accuracy and precision of the assay.
- Document all quality control results and ensure compliance with assay specifications.
- Results Interpretation:
- Interpret the quantitative results based on established reference ranges or clinical guidelines.
- Consult with a qualified healthcare professional for result interpretation and patient management decisions.
- Reporting:
- Generate a comprehensive report including sample identifiers, analyte concentrations, and any relevant notes or observations.
- Maintain accurate records of all assay results, including raw data and analysis parameters.
- Storage and Disposal:
- Store the kit components according to the recommended conditions specified in the user manual.
- Dispose of all waste materials in accordance with local regulations for biohazardous and chemical waste.
- Troubleshooting:
- Refer to the troubleshooting section of the user manual for guidance in case of any technical issues encountered during the assay.
- Feedback:
- Provide feedback to the manufacturer regarding the performance of the kit, including any suggestions for improvement or concerns.
- References:
- Refer to relevant literature and publications for additional information on biogenic amines analysis and LC-MS/MS methodology :
Stabilization of urinary biogenic amines measured in clinical chemistry laboratories
- Disclaimer:
- This protocol serves as a general guideline and may require optimization based on specific laboratory conditions and equipment.
Note: Always adhere to the instructions provided in the user manual accompanying the Biogenic Amines Urine LC-MS/MS Kit and consult with qualified personnel or technical support if needed.